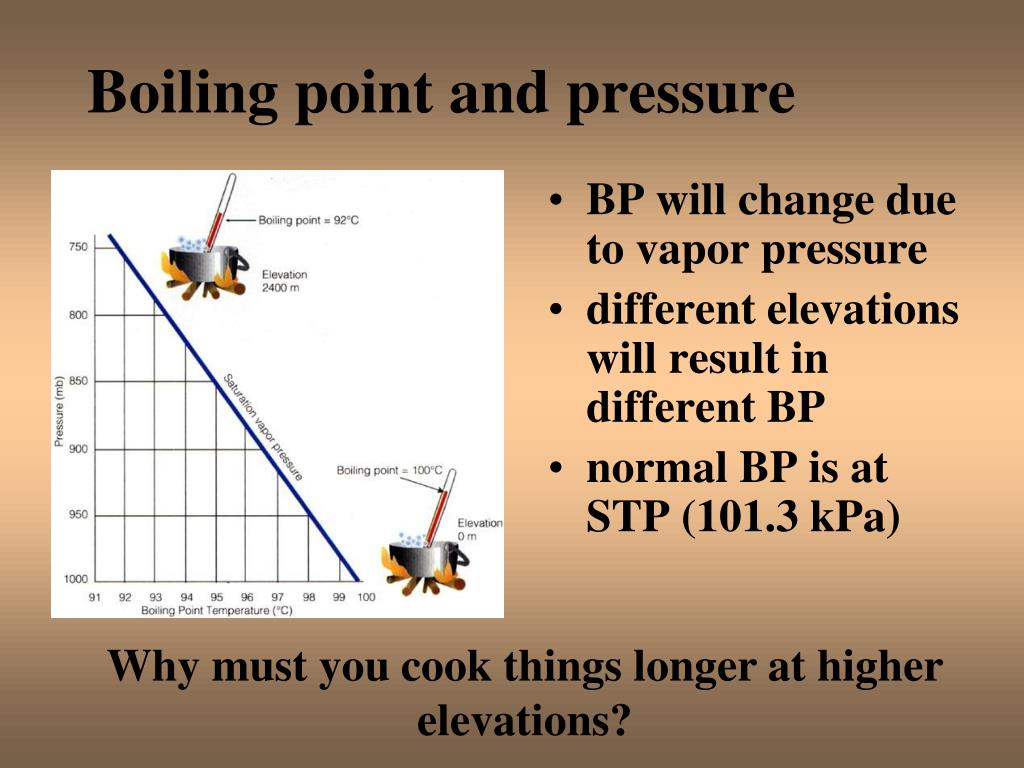
What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid . the pressure of gas above a liquid affects the boiling point. factors that affect the boiling point. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is equal to the. Less than one atmosphere, the boiling point of the liquid is lower. In an open system this is called atmospheric pressure. When the external pressure is: As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by.
from www.slideserve.com
When the external pressure is: boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. In an open system this is called atmospheric pressure. the pressure of gas above a liquid affects the boiling point. factors that affect the boiling point. Less than one atmosphere, the boiling point of the liquid is lower. the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is equal to the.
PPT Energy, Temperature, Phase Changes PowerPoint
What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. Less than one atmosphere, the boiling point of the liquid is lower. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is equal to the. When the external pressure is: boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. the pressure of gas above a liquid affects the boiling point. In an open system this is called atmospheric pressure. the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at. factors that affect the boiling point. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid In an open system this is called atmospheric pressure. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is equal to the. the boiling point is. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. When the external pressure is: Less than one atmosphere, the boiling point of the liquid is lower. In an open system this is called atmospheric pressure. the pressure of gas above a liquid affects the boiling. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From socratic.org
What is the relation between critical temperature and boiling point or What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid the pressure of gas above a liquid affects the boiling point. factors that affect the boiling point. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. When the external pressure is: boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. the boiling point is the temperature at which the vapor pressure of. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid factors that affect the boiling point. the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. As the temperature of a liquid increases, the vapor pressure of. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From mungfali.com
Vapor Pressure And Boiling Point Relationship What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid Less than one atmosphere, the boiling point of the liquid is lower. When the external pressure is: the pressure of gas above a liquid affects the boiling point. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is equal to the. boiling point of a liquid can be. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From mungfali.com
Water Boiling Point Pressure Chart What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid factors that affect the boiling point. the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. As the temperature of a liquid increases, the vapor pressure of. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From www.worksheetsplanet.com
What is Boiling Point What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. In an open system this is called atmospheric pressure. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. When the external pressure is: Less than one atmosphere,. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From bceweb.org
Water Boiling Point Vs Pressure Chart A Visual Reference of Charts What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid Less than one atmosphere, the boiling point of the liquid is lower. the pressure of gas above a liquid affects the boiling point. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is equal to the. the boiling point is the temperature at which the vapor pressure of. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid factors that affect the boiling point. In an open system this is called atmospheric pressure. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is equal to the. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid Less than one atmosphere, the boiling point of the liquid is lower. the pressure of gas above a liquid affects the boiling point. When the external pressure is: the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at. boiling point of a liquid can be increased by increasing the. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From www.youtube.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid When the external pressure is: As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at. at a basic level, the boiling point of a substance is the temperature at which its. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From mavink.com
Water Boiling Point Vs Pressure Chart What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid In an open system this is called atmospheric pressure. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. When the external pressure is: boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. the boiling point. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From mavink.com
Water Boiling Point Pressure Chart What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. In an open system this is called atmospheric pressure. the pressure of gas above a liquid affects the boiling point. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid Less than one atmosphere, the boiling point of the liquid is lower. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. the pressure of gas above a liquid affects the boiling point. the boiling point is the temperature at which the vapor pressure of. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. Less than one atmosphere, the boiling point of the liquid is lower. at a basic level, the boiling point of a substance is the temperature at which its vapor pressure is equal to the. In an open system this is. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From www.slideshare.net
Lecture 13.2 Liquids What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid When the external pressure is: As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external. In an open system this is called atmospheric pressure. the boiling point is the temperature at which the vapor pressure of the liquid equals the pressure at. at a basic level, the boiling point. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.
From exomkbouq.blob.core.windows.net
What Is Boiling Point Elevation Constant at Ronnie Moore blog What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid In an open system this is called atmospheric pressure. boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by. factors that affect the boiling point. Less than one atmosphere, the boiling point of the liquid is lower. When the external pressure is: the pressure of. What Is The Relationship Between External Pressure And The Boiling Point Of A Liquid.